 Let's
answer this question in several parts. First, Let's
answer this question in several parts. First,
Why are snow crystals six-fold symmetric?
The hexagonal symmetry of snowflakes originates with the
underlying symmetry of the ice crystal lattice. Water molecules hook up in
a hexagonal lattice (see the
Snowflake Primer), and the molecular symmetry is imparted to the snow
crystal form via faceting (see
Crystal Faceting for more on how this works).
In particular, tiny snow crystals are usually in the form of small
hexagonal prisms (see the
Snowflake Primer), which is how the six-fold symmetry of snowflakes gets
its start.
Second, let's ask
Why do snow crystals have
such complex shapes?
If faceting always dominated snow crystal
growth, then snow crystals would always be shaped like simple hexagonal
prisms. Faceting does dominate when the crystals are very small, or when
the growth is very slow. But larger crystals tend to branch out, through
something called the branching instability, which is described in
some detail at
Snowflake Branching. Instabilities like this often produce complexity
in nature -- the complex fluttering motion of a flag in the wind and the
complex motion of waves breaking on the beach are other examples of
instabilities in nature producing complexity.
Finally, let's look at the original question by
following the life story of a complex symmetrical snow crystal.
The growth usually begins up in a cloud with a minute dust particle,
which provides a structure on which water molecules can start condensing to
form a snow crystal. When the crystal is very small, faceting dominates the
growth, and the crystal quickly grows into a simple hexagonal prism.
As the crystal grows larger, the corners of the hexagon stick out a bit
further into the supersaturated air and thus grow a bit faster. The
slightly faster growth at the corners soon causes the hexagon to sprout arms
(see
Snowflake Branching). And since the ambient atmospheric conditions are
nearly identical across the crystal, all six budding arms grow at roughly
the same rate.
The temperature seen by the snow crystal is not constant in time,
however, since the crystal is being blown about and is thus carried over
great distances in a cloud. But the crystal growth rates depend
strongly on temperature (as is seen from the
morphology diagram). Thus the six arms of the snow crystals each change
their growth with time, reflecting the ever-changing conditions in the
cloud. And because each arm sees the same conditions, each arms grows the
same way.
 So that's the story. The intricate shape of a single arm is determined by
the ever-changing conditions experienced by the crystal as it falls.
Because each arm experiences the same conditions, however, the arms tend to
look alike. The end result is a large-scale, complex, six-fold symmetric
snow crystal. And since snow crystals all follow slightly different paths
through the clouds, individual crystals all tend to all look different.
So that's the story. The intricate shape of a single arm is determined by
the ever-changing conditions experienced by the crystal as it falls.
Because each arm experiences the same conditions, however, the arms tend to
look alike. The end result is a large-scale, complex, six-fold symmetric
snow crystal. And since snow crystals all follow slightly different paths
through the clouds, individual crystals all tend to all look different.
Click on image to view. |
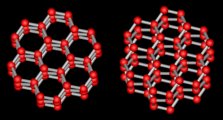 A crystal is a material for which the molecules inside are all lined up in a
specific way called the crystal lattice. The water molecules in
ice form a hexagonal lattice as shown at right (two views of the same
thing). Each red ball represents an oxygen atom, and the grey sticks
represent hydrogen atoms. There are two hydrogens for each oxygen, making
the usual H2O.
A crystal is a material for which the molecules inside are all lined up in a
specific way called the crystal lattice. The water molecules in
ice form a hexagonal lattice as shown at right (two views of the same
thing). Each red ball represents an oxygen atom, and the grey sticks
represent hydrogen atoms. There are two hydrogens for each oxygen, making
the usual H2O. Let's
answer this question in several parts. First,
Let's
answer this question in several parts. First,
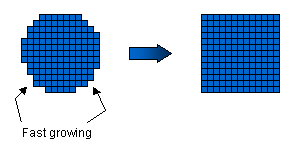 Water
molecules from the air can readily attach to these rough surfaces, which
thus grow relatively quickly. The facet planes are special, however, in
that they tend to be smoother on a molecular scale, with fewer dangling
bonds. Water molecules cannot so easily attach to these smooth surfaces,
and hence the facet surfaces advance more slowly. After all the rough
surfaces have grown out, what remains are the slow-moving facet surfaces.
The picture at right shows the idea for a crystal with four-fold symmetry
(which is easier to draw).
Water
molecules from the air can readily attach to these rough surfaces, which
thus grow relatively quickly. The facet planes are special, however, in
that they tend to be smoother on a molecular scale, with fewer dangling
bonds. Water molecules cannot so easily attach to these smooth surfaces,
and hence the facet surfaces advance more slowly. After all the rough
surfaces have grown out, what remains are the slow-moving facet surfaces.
The picture at right shows the idea for a crystal with four-fold symmetry
(which is easier to draw).
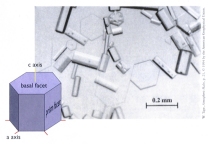
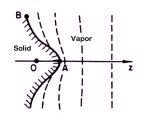 The answers to these questions lie in just how water
molecules travel through the air to condense onto a growing snow crystal.
The water molecules have to diffuse through the air to reach the
crystal, and this diffusion slows their growth. The farther water molecules
have to diffuse through the air, the longer it takes them to reach the
growing crystal.
The answers to these questions lie in just how water
molecules travel through the air to condense onto a growing snow crystal.
The water molecules have to diffuse through the air to reach the
crystal, and this diffusion slows their growth. The farther water molecules
have to diffuse through the air, the longer it takes them to reach the
growing crystal.