|
|
|
|
Science 11 December 2009: |
News Focus
Solar Fuels:
Sunlight in Your Tank
Conventional solar technologies produce electricity, but most transportation fuel comes from oil. A new class of solar chemical reactors aims to make liquid fuels from air, water, and sunshine.
|
Just not quite yet. At the moment, Diver's reactor is in
pieces all around the garage. In the dust covering
one of the mirrors is scrawled "I
 solar" and
"Hi Dad," written by Diver's daughter Tracy during a
recent visit. Diver disassembled the reactor after a
recent test run that showed that iron oxide catalysts
worked efficiently to split water. But the temperatures got
so high, and the temperature changes so dramatic, that
they fractured many of the flat, 1-cm-square catalyst
tiles and cracked the ceramic housing holding them in
place.
solar" and
"Hi Dad," written by Diver's daughter Tracy during a
recent visit. Diver disassembled the reactor after a
recent test run that showed that iron oxide catalysts
worked efficiently to split water. But the temperatures got
so high, and the temperature changes so dramatic, that
they fractured many of the flat, 1-cm-square catalyst
tiles and cracked the ceramic housing holding them in
place.
Back in town at the Albuquerque Marriott, Diver and about 30 of his colleagues from around the world have spent most of the past 2 days huddled in conference rooms searching for solutions to these sorts of problems and comparing notes on different versions of the technology.* They've sorted through white-boards full of technical challenges with their solar reactors, ranging from boosting the speed at which the catalysts work to keeping the windows on the reactors clean so the focused sunlight can stream in.
The good news is that the technology has been shown to work on a small scale, producing tens to hundreds of liters of energy-rich gases per hour. "This is not a pipe dream," says Alan Weimer, a chemical engineer and solar-fuels expert at the University of Colorado, Boulder. "We have things that actually work." And a simpler version of the technology is already close to market (see sidebar, p. 1474).
But there's bad news, too. For now, the process is costly and unlikely ever to compete with producing gasoline from fossil fuels without a price on carbon emissions. "We are not at the point where it is feasible on a commercial scale," Weimer says. Diver agrees: "It puts us in this in-between land." This awkward stage of development is a crowded territory known as the "valley of death" among venture capitalists, who specialize in turning promising technologies into moneymaking businesses. Diver and his colleagues have assembled here in hopes of finding a way out of this valley. Their immediate goal has been to put together a white paper that lays out the case for solar fuels—more officially known as solar thermochemical fuels, because heat from concentrated sunlight drives the needed chemical reactions.
This search for the way out of the valley of death has become the quintessential problem facing energy researchers of all stripes today. Whether they work in a relatively sparsely populated area such as solar fuels or in cellulosic ethanol, which is widely hailed to be the future of ethanol technology, they must develop a new technology for producing fuel or electricity. And they must do it cheaply enough to compete with fossil fuels, a technology that engineers have been whipping into shape for more than 100 years and that has trillions of dollars of infrastructure installed to make it work.
At a break during the meeting here, Diver is chatting with Robert Palumbo, a mechanical engineer at Valparaiso University in Indiana. "Is what you are doing harder or easier than landing a man on the moon?" Palumbo asks. "Harder," Diver says without hesitation. "They only had to do it once." Besides, he adds, "they didn't have to worry about economics."
NASA engineers were also the beneficiaries of a sustained financial push to develop the technology. Not so with solar thermochemical fuels. The field got an initial boost after the Arab Oil Embargo of the early 1970s raised concerns about rising oil prices and Western countries' dependence on Middle Eastern oil. Groups in Europe, the United States, and Japan spent a few years searching for catalysts to convert heat into fuels regardless of the heat source, but interest had largely died out by 1983 as oil prices again plunged. Solar-fuels programs in Switzerland and Germany took off again in the early 1990s, driven by growing concerns over climate change and fears that world oil production was nearing its peak. Beginning in 2003, a brief bolus of funding for producing hydrogen fuel kick-started several efforts in the United States, including the Sandia effort, but that funding has since disappeared.
Despite the ebbs and flows of funding, attendees at the Albuquerque meeting argue that the need for solar-fuels research is only growing. Anxiety about rising atmospheric CO2 levels—now at 385 parts per million, up 43% from preindustrial levels—"is the underlying premise of why we are here," says James Miller, a materials scientist at Sandia. Miller helps run the lab's Sunshine to Petrol program, which is now supported by internal Sandia funding. Energy security is another concern. With 58% of U.S. oil now coming from imports, Miller notes, "the transportation sector is very vulnerable to disruptions in supply."
But a potential plus for solar thermochemical fuels is one
that renewable-energy providers rarely mention:
scalability. Energy use occurs on a scale unlike any
other. In 2006, for example, humans used 472
quadrillion BTUs (quads) of energy, 21% of it in the
United States. More than
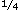 of the U.S.
portion went to transportation, and nearly all of
that came from oil. As a result, any new technology
hoping to dethrone oil drilling will have to supply
not only cheap energy but a lot of it. That's where
most renewables hit the skids.
of the U.S.
portion went to transportation, and nearly all of
that came from oil. As a result, any new technology
hoping to dethrone oil drilling will have to supply
not only cheap energy but a lot of it. That's where
most renewables hit the skids.
Take biofuels. In 2007, Congress mandated that by 2022 the United States will make an estimated 117 billion liters of ethanol. Producing it is expected to require anywhere from tens of millions to hundreds of millions of hectares of land, depending on future efficiency increases in turning biomass into ethanol. Even so, the 2022 ethanol supply is expected to meet only about 13% of U.S. demand for transportation fuel. With biofuels already increasing the pressure on agricultural and conservation reserve lands, it's hard to imagine the figure approaching 100%.
That's where concentrated solar-fuel plants have an important advantage, says Ellen Stechel, who heads Sandia's Fuels and Energy Transitions Department. Because the technology uses the full solar spectrum and concentrates sunlight, it uses far less land than biomass, photovoltaics, and other technologies that also rely on capturing sunlight. That could make it far more straightforward for the technology someday to supply a large fraction of transportation fuels.
At the Albuquerque meeting, Robert Wegeng, a mechanical engineer at the Pacific Northwest National Laboratory in Richland, Washington, walked attendees through an initial calculation that shows the potential of an intermediate, and simpler, version of solar-fuel technology. Wegeng is developing a solar reactor capable of converting natural gas to a mixture of hydrogen and carbon monoxide—known as synthesis gas—that can be used to make liquid fuels.
Wegeng calculates that a solar-fuel plant made up of an array of 10,000 parabolic dishes would take up only a few square kilometers of land. Yet it could capture 1 gigawatt of energy, enough to upgrade natural gas equivalent to 2 million gallons of gasoline per day or 700 million gallons per year. "Twenty such plants would offset U.S. petroleum exports by 10%," Wegeng says. And there's plenty of desert land to scale the technology up even further.
"This can potentially make as much fuel as we want," Stechel says. Even better, because the technology can be used as the first step to producing liquid hydrocarbons, it would allow countries to continue to use their built-up gasoline-based infrastructure without having to rely on mining fossil fuels. Wegeng and others at the Albuquerque meeting were quick to add that they don't think solar fuels should be considered as an alternative to biofuels. "There is no silver bullet when it comes to replacing fossil fuels," Palumbo says. Instead, solar thermochemical fuels are one of the pieces of "silver buckshot" needed to do the job. Stechel agrees. "It's another good option," she says. "We need them all."
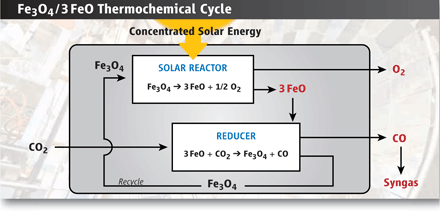
In reality, solar thermal fuel technologies are several pieces of buckshot, racing toward the same target: the simple ability to split water or CO2. These compounds can be split directly with heat, but they can create an explosive mixture of gases that need to be separated. By using catalysts, researchers can reduce the temperature of their reactors and produce H2, CO, and O2 in separate steps, eliminating the separation problem. Take Diver's setup, at least when it's not scattered in pieces around the garage. Inside the reactor sit 14 cobalt ferrite rings, made from a mixture of cobalt and iron oxide, or rust. Each ring is about 1/3 of a meter in diameter and rotates between two separate reaction chambers. Sunlight from the heliostat and the parabolic mirror is focused through a window in the reactor, heating the interior to as high as 1500°C. At that temperature, the flakes of iron oxide catalyst on the outside of each ring spit out oxygen molecules, which are vented to the outside (see figure, below). As the disks turn, they enter the second, 1100°C chamber, which is spiked with CO2 or water vapor from which the catalyst swipes oxygen atoms. This reaction generates the energetic gases (H2 or CO), which are collected, and restores the catalyst to its original composition, ready for another turn of the wheel.
For now, however, none of these catalysts is good enough. Although the iron oxides can give up a large percentage of their oxygen atoms in the first, high-temperature step, they melt at 1800°C, just above the typical temperature in the reactors. And when they cool, they form an inert slag that is far less reactive in subsequent catalytic cycles. The iron oxide portion is also brittle and unstable. So it is typically mixed with large quantities of inert ceramics, which must be heated even though they don't perform any catalytic work. Moreover, because the ferrites are relatively large chunks of solid material, not all of the catalytic material in the center of each flake participates in the reaction.
|
Round
and round. In this reactor, heat from
concentrated sunlight converts a magnetite (Fe3O4)
catalyst into wüstite (FeO) and oxygen. A second
step swipes oxygen to restore the catalyst.
CREDIT: (PHOTO CREDIT) RANDY MONTOYA/SANDIA |
 |
|
For now, the Swiss group has the best reported efficiency in converting heat from incoming sunlight into fuel, at just over 3%. For the technology to have a shot commercially, however, solar-fuels researchers say they will need to do significantly better. Solar electric generators are already 20% efficient at converting sunlight to electricity. And current commercial electrolyzers can convert electrical energy to hydrogen gas at 65% efficiency. By combining the two devices, engineers can convert sunlight into hydrogen at more than 13% efficiency with conventional technology. "We're trying to do it more directly and more efficiently," Miller says.
There's reason for optimism, Miller adds: The theoretical maximum efficiency of converting heat to chemical bonds is 75%, limited by the Carnot efficiency of the heat engine. For a successful catalyst, he says, "we only have to get to about half of that," probably resulting in an overall reactor efficiency of slightly more than 20%. "A factor of less than 10 improvement certainly seems manageable," Miller concludes.
Others agree and suggest that the goal for the field should be a reactor that converts 20% of incoming heat to fuel by 2020. Better efficiency will have other payoffs as well. Most important, it could enable engineers to use smaller concentrators and heliostats, which account for roughly half the cost of current solar thermal systems.
So how much would fuel from concentrated sunlight likely cost? "We are confident we can get this number down to less than $10 for a gallon of gas," Miller says. At that price, Steinfeld says, "it's clear solar fuels will have a hard time competing directly with fossil fuels." But if the world begins to move away from fossil fuels to CO2-neutral fuels, Steinfeld says he's convinced that solar thermochemical reactors could step in to meet the demand.
Improving catalyst efficiency and getting the price down won't be the only hurdles solar-fuels researchers have to leap. Catalyst lifetimes in the reactors need to improve. And researchers will need to find a way to run the reactors on stored heat at night so that future commercial plants can run 24/7, typically an industry and financial requirement. But even though that list sounds daunting, solar-fuel researchers insist that most of the items are fairly standard engineering challenges that will be overcome with sustained work on the technology. "There is every reason to believe there are no showstoppers here and it will be viable," Stechel says. The question now, and the hardest challenge ahead, is whether the field's successes to date and promise for the future will persuade funding agencies to put them on the path to commercial reality.
* Workshop on Solar Thermochemical Cycles, 2–4 November 2009, Albuquerque, New Mexico.
T
-
Biomass Fuel Starts to See the Light
Robert F. Service Even with a major push, commercial plants capable of turning CO2 or water into liquid fuels are still likely to be 2 decades away. A simpler version of the technology, however, already appears headed to market.
Even with a major push, commercial plants capable of turning CO2 or water into liquid fuels are still likely to be 2 decades away. A simpler version of the technology, however, already appears headed to market. Sundrop Fuels Inc., an energy start-up based in Louisville, Colorado, recently commissioned a 1-megawatt solar array to convert wood waste and other forms of biomass into a gaseous blend of carbon monoxide and hydrogen—known as synthesis gas—that can be converted into gasoline. The plant uses an array of 2700 mirrors to concentrate sunlight on a 20-meter-tall solar tower to produce heat needed to drive the chemical reactor. The company has told Science that in 2012 it intends to open a commercial plant capable of capturing 60 megawatts and use that energy to produce 19 million liters of gasoline annually.
CREDIT: SUNDROP FUELS INC. "This is a very good development," says James Miller, a materials scientist at Sandia National Laboratories in Albuquerque, New Mexico, who helps direct the lab's Sunshine to Petrol program. "If this is successful, it will help demonstrate that solar thermal energy will be an important piece of how we produce liquid transportation fuels in the future."
At a recent workshop on solar fuels, participants spent considerable time debating whether the small solar-fuels research community should push for large-scale demonstration plants akin to Sundrop's. Such plants offer a way to take a carbon source, such as biomass or coal, and increase its energy content while converting it into a liquid hydrocarbon. "On the one hand, it's a distraction" from the ultimate goal of converting CO2 to fuel with sunlight, says Ellen Stechel, who heads Sandia's Fuels and Energy Transitions Department. "But on the other hand, it's a steppingstone to where we want to be."
That steppingstone is easier to reach because it employs a simpler chemical reactor. Splitting CO2 and water requires specialized catalysts that must be transferred between two separate stages to carry out different reactions. Converting biomass to syngas, by contrast, requires only quickly heating the biomass in the presence of steam. Biomass gasification in conventional chemical reactors has been explored for decades. But the technology has been held back in part because some of the biomass itself, or other fossil fuels, must be burned for heat to power the reaction. Getting the heat from solar concentrators eliminates that heating cost and avoids generating excess CO2. But how the initial cost of the solar concentrators affects the economics of the process remains to be seen. "Building a demonstration plant gives you the opportunity to work some of those issues out," Miller says.
In related work, researchers around the globe are investigating using heat from concentrated solar energy to convert methane in natural gas into liquid fuels, turn limestone into cement, and reduce metal oxides into metals. These industrial processes currently use massive amounts of energy and emit copious quantities of CO2. Jane Davidson, a mechanical engineer at the University of Minnesota, Minneapolis, says solar thermal systems are ready to take this first big step. "This is realistic," Davidson says. "This can happen, and it can make a big difference."