back to Stopdown, home page, click here
back to Science, home page, click here
| Atoms, molecules, cells, organisms | |||
atoms combine to make molecules, molecules combine to make cells, cells combine to make organisms
| An atom, nucleus and electrons. | Atoms join to make a molecule. | A cargo protein (a molecule) carries a "vesicle" full of molecules for delivery. | |||||
As pictured above, left, an atom has a nucleus which is heavy and in the middle, and electrons which fly around it, held in orbit because the nucleus is electrically "positive" and the electrons are electrically "negative" Positive and negative attract. There is a gravitational pull between electron and proton, as with the earth orbiting the sun, but it is very slight. Like all molecules, life molecules: sugars, fats, proteins, vitamins, DNA and others, are made from atoms combining. Atoms combine by sharing electrons in a way that takes a kind of strain off of both atoms. One atom partially gives up an electron to the atom it bonds with, and this shared electron is the bond we call a chemical bond. (Sometimes the bond is between the same kind of atom such as O2, two oxygen atoms combined as in oxygen we breath.Molecules combine with other molecules by the same process: atoms on the outside of one molecule bond, by sharing electrons, with atoms on the outside of another molecule, so that the two molecules become attached..... Life chemistry has a lot of clever processes carried out by molecules, such as the delivery system shown, above right: a cargo protein walks, one foot in front of the other, carrying a round bundle full of molecules for delivery to where they are needed. The molecule walks along a "microtubule", which is a catwalk inside a cell .................Below, left, is a cell, the basic unit of life, the simplest fully living thing. It eats, reproduces, has complex internal organs, talks to other cells with proteins and more. It is made of molecules, some acting as internal and external messengers, some forming the outer membrane, some carrying out the burning of fuel chemicals, and much more. Photosynthesis, the capture of the energy in light to fuel the growth of a plant, is carried out inside plant cells, which consume carbon dioxide and release oxygen, whereas animal cells consume oxygen and release carbon dioxide. The captured energy in photosynthesis is the basis of life's energy. Cells build molecules for information, function, and fuel in an unpward building process called "anabolic" chemistry. "Burning" down to smaller molecules is called a "catabolic" reaction. Reactions that release energy fuel the upward building reactions and other processes. Cells reproduce by dividing into two cells, a process called mitosis. Independent cells are usually in a colony, like bacteria, and some view such colonies as organisms...........Organisms, such as the plankton species, below, right, are made of cells. An organism is conceived when two cells come together from parents, forming one cell which then divides into two, which divide into four, then to eight and so on. From the beginning the shape of the cell aggregate is very orderly. As the cells divide they "differentiate", they become different according to the type of tissue they are forming, such as blood, bone, nerves, muscle etc. Their DNA, which codes protein production, remains the same, but it is "expressed" differently, sort of like a software program that can make changes to facilitate different applications. In the plankton figure below (right), which is magnified, you would be able to see cells, like the cell on the left, with a microscope that greatly magnified a section of the organism. .........
| The cell is an organism, a living thing. It eats and excretes, | Organism (Plankton) is made up of cells. | |||||||||
| reproduces by dividing, receives and sends messages, reads its | The cells are too small to see here. A higher | |||||||||
| surroundings chemically, moves with intent. | magnification of a section would show cells. |
The four levels outlined above are outlined a little better below.
(1) Atoms and electron bonds between atoms.
Below, left An atom is pictured with the nucleus, a collection of protons, in the center, and the little beads of fire flying around it are electrons. Electric attraction between the "positive" nucleus and "negative" electrons holds the electrons in orbit like gravity keeps planets circling the sun. The more electrons there are in an atom, the more protons there must be to hold the electrons in orbit. (Small discrepensies occur, positive and negative ions.) In the atom the nucleus, pictured as a sphere, we know contains as many protons because there are many electrons. The nucleus also contains "neutrons" which have no effective charge, (neutral) and so don't have any influence on the electrons. Neutrons are massive, like a proton. And they have the beguiling property of being able to turn into a proton by releasing an electron. The neutron, therefore, is not absent of charge but is doubly charged -- with the resulting cancellation that makes it neutral. A neutron "weighs more" than a proton (very slightly) because of the electron it contains that the proton doesn't.
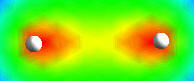
Below, right, Two atoms are shown unbonded, and bonding with their electrons, shown by the yellow clouds merging to the center. These clouds are called "electron clouds" or "probability clouds" because the electron moves so fast that the best way to depict it is as a cloud showing where is is likely to be. ( The red and green depict the nuclear and electromagnetic fields, perhaps, we're not sure. Image from web. All were talking about is the nucleus (the sphere) and the electron.) The issue is really more complex because the electron, a particle when in the form of static electricity (such as comes off a rug and shocks you when you touch metal) is not a particle when it is moving fast in its atomic orbit. Its high velocity turns it into a wave. The particle to wave change at high velocity is observed with electrons and other particles at high velocity in the laboratory. The bond shown is the simplest bond there can be between atoms. But as the atoms get bigger (more electrons and protons) the clouds and become more complicated, and as do the bonds.
| A multi-electron atom | two hydrogen atoms | electron bond making H2, a simple molecule, hydrogen gas. | |||
|
|
 |
||||
|
|
|||||
A chemical bond happens because some atoms are inclined to give electrons and others are inclined to accept electrons in order to have a more stable electron arrangement. (We intend to discuss this in other basics sections.) Electrons also want to be in pairs, so unpaired electrons seek out unpaired electrons on other atoms, making a "bonding pair." The bonding pair will usually be drawn in to one atom and partially let go of by the other, but the electrons are still shared.
(2) Molecules
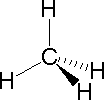
Life chose carbon, which naturally makes four bonds, as the backbone of life chemistry. As shown in the carbon-based molecules below four bonds makes nice chains. In methane, lower left, the four hydrogen atoms are as far apart as they can get in space. If you compare three bonds and five and six with four -- using balls of clay and sticks, you will see that four takes in all angles, and yet is nicely simple. It is like the U-joint in a car: flexible, balanced, simple and strong. Three is more limited in the angles it takes in, and, after bonding two ways to make a chain, has only one electron free for side bonding. Five is interesting, but complicated and asymmetrical. Six is orderly but not flexible, and it is crowded. Four is the one that works best.. Question: Would life have found a way if there was no carbon? No carbon, no life?
Below, left, three figures depicting carbon bonded to four hydrogen atoms (which makes a gas molecule called methane), showing how carbon's four bonds are positioned (usually). To the right are simple carbon/hydrogen molecules (all gasses like methane) which give an idea of what carbon chains look like.
 |
 |
|||||||||||||||
| methane | methane | methane (one carbon) | ethane (two carbons) | propane (3 carbons) | butane (4 carbons) |
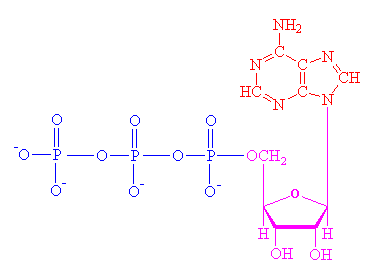
Molecules are characterized by pictures that approximate their structure. Below are two renderings of adenosine triphosphate, or ATP, which is a universal fuel molecule in life chemistry. When it releases one of its three phosphate groups, which are on the left (phosphorus bound to three oxygens) it releases energy that is used to make another chemical reaction happen........It is not that hard to learn to read a structure like that on the left. Carbon, nitrogen, hydrogen, oxygen and phosphorus (types of atoms) are shown by their initials. On the sugar (the middle part, in magenta color) the corners of the ring mark a carbon atom not shown with a letter C. In diagrams of organic molecules, a corner means carbon atom. Where there are two connecting lines there are two bonds of two electrons, called a double bond. The minus signs on the oxygens, lower left, mean that each of those oxygens is carrying an extra electron, so they each carry a negative charge.
 |
 |
| The ATP molecule is composed of three components. Below in
pink is a sugar molecule, ribose (the same sugar
that forms the basis of DNA). Attached above is a base (the group
consisting of linked rings of carbon and nitrogen atoms); in this case the base
is adenine. To the left of the sugar is
a string of phosphate groups. These phosphates are the
key to the activity of ATP. ATP is like a match. To strike the match and give
energy to cause another reaction, one of the three phosphate groups is
removed by another molecule, usually called a kinase. For each step of the
cargo protein a molecule of ATP is "burned" to give adenosine di-phosphate,
or ADP. To reload the match a phosphate group is added to a molecule of ADP,
making ATP again. ............................................................................................................. |
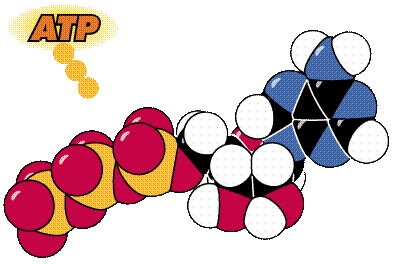
This is the same molecule, different model. The colors don't
match the version on the left, but the orientation is basically the same:
phosphate groups on the left, the ribose sugar in the middle, and the
adenine base above the sugar.
......................................................................................................... |
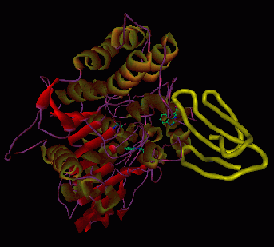
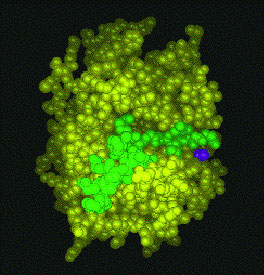
Below are large molecules, in this case proteins or RNA. Some of these pictures may be of one molecule bonding with another (Sorry, we need to replace these with identifiable species. We neglected to make notes on these internet images.) In the molecules on the left top and left bottom the atoms are depicted as little connected spheres (except for the red ribbon upper left). In the other images the ribbons and strands represent lots of atoms strung together, and detail is sacrificed -- individual atoms are not shown. Molecules are visualized scientifically by crystallizing a pure sample of a chemical and shining x-rays through it. The emerging X-rays are deciphered by computer programs. .....Unlike a cell, individual atoms and molecules can't be seen in a microscope, they are too small.
|
 |
||||
| Top and bottom: large proteins (molecules) | |||||
 |
|
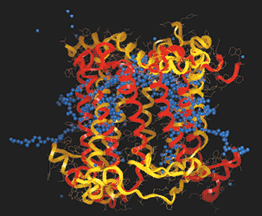
Below are depictions of interactions between large molecules, such as DNA and proteins like those shown above. As can be seen molecules have complex reaction interfaces.
 |
|||||||||
(3) Cells
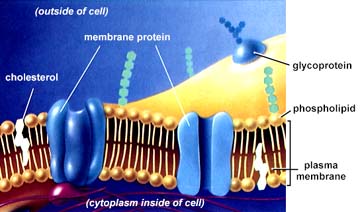
Molecules combine to make cells, which have an outer membrane and internal organs. Cells eat, excrete and multiply, and can often navigate by following chemical signals and propelling themselves with flagella (see cell, below middle, "flagellum") and other means. T-Cells, below left, are communicating at their surfaces, forming a synapse, a chemical communication grid between cells. Nerve cells also communicate at a synapse. Below middle, the anatomy of a cell. Below right, a cell membrane, showing pores where cells take in and release chemical messengers (proteins), and where nutrients are taken in and waste released.
 |
 |
 |
Life inside a cell is a complex event..... Below, left is a cargo protein carrying a load of chemicals in a vesicle by walking along a microtubule -- catwalks inside cells. The cargo protein gets its energy by burning the cellular fuel ATP (see above). And so it walks, one foot in front of the other, like a person. (Inchworm type of locomotion, in which both feet can stay connected to the microtubule, was considered possible, but walking, in which feet have to let go and reattach, seems to be what is happening.)..........Below right. The "vesicle" which contains proteins for transport from one organelle to another, is depicted budding away from one organelle and fusing with another. In this picture the cargo protein and microtubule are not shown. Question... what are the methods of vesicle transport other than cargo proteins on a microtubule?
| Carry the load: a "cargo protein" walks: one foot in front of the other. | |||
(4) Organisms
for a web page describing microbes click here
Organisms are made from cells, and they are made essentially in the image of a cell, with an outer skin, ways to take in and let out food and signals, a complex set of internal organs and internal communication techniques, systems of reproduction. Below, left, is an egg which, rather than hatch, starts to form an organism by creating an internal chamber which will become the equivalent of a stomach........Below, right, starfish larvae. (baby starfish)
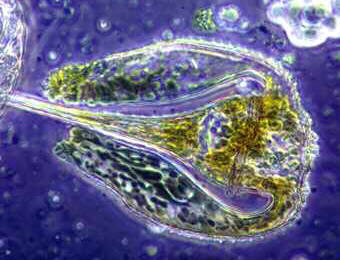
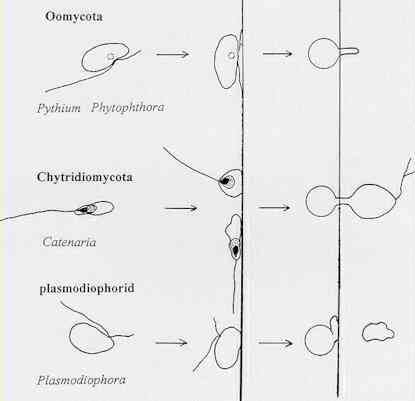
Organisms. On the left, below, plankton (?). On the right below fungal spores penetrating a membrane or other barrier. We would like at develop a page on what funguses (fungi) are.
 |
 |
||||
Below: more microorganisms.
| .. |
Below, more organisms.
| plankton | plankton | plankton |
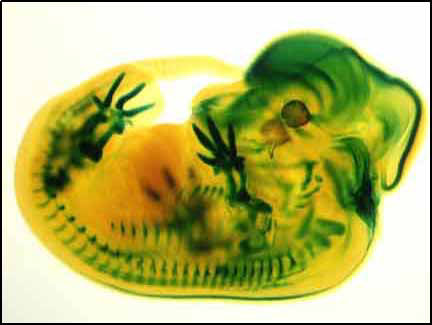
Below: mouse embryo. The Genes of a mouse are pretty close to that of a human. Chimpanzees and humans are very close, genetically.
back to Stopdown, home page, click here
back to Science, home page, click here